Among the possible systems investigated for energy production with low environmental impact, fuel cells are very promising as electrochemical power sources both for stationary energy production and application in portable technology and electric vehicles. Polymeric electrolyte membrane fuel cells (PEMFCs) are the most promising candidates for the latter application.
The material most widely used forelectrolyte membranes is Nafion, a perfluorinated sulfonated polymer (Figure 1). Because of its water assisted conduction mechanism, Nafion can be used only at temperatures below 100 °C [1]. For practical applications, however, operating PEMFCs at higher temperature is desired, both for hydrogen and methanol-fuelled cells. When hydrogen is used as a fuel, an increase in the cell temperature above 100°C produces enhanced CO tolerance, faster reaction kinetics, easier water management, and reduced heat exchanger requirement. The use of methanol as a fuel for vehicles has several practical benefits such as easy transport and storage, but the slow oxidation kinetics of methanol and its crossover through the membrane reduce the efficiency of direct methanol fuel cells (DMFCs). Therefore, increasing the operation temperature of proton conducting membranes is a key issue in the development of PEMFC technology [2,3].
 The strategy followed by researchers of NAST Centre is to develop organic/inorganic hybrid membranes in order to achieve the goal of obtaining PEMs capable to operate at temperatures up to and above 120°C . Organic/inorganic hybrids are investigated for a variety of applications, ranging from optics to electronics, to sensors and many others, since they offer the possibility to combine the properties of the two components in a unique material [4]. Thus, we have studied nanocomposites where inorganic materials were added to a polymeric matrix, either of Nafion or of totally aromatic sulfonated polymers. In the case of polymeric electrolytes, the addition of nanocrystalline oxides is effective in improving ionic conductivity, mechanical strength, and thermal stability [5]. Since performance is strongly influenced by the filler particle size, we developed a simple and versatile route for the preparation of ceramic oxides with nanometric grain size [6]. MxOy (M = Ti, Nb, In and Zr) were synthesized by rapid hydrolysis of an alcoholic solution of metal alkoxides. The preparative conditions were optimized to enhance the reaction kinetics leading to fast nucleation and favoring the formation of nanoparticles. Figure 2 shows the TEM micrograph of TiO2 (chosen as a representative example) obtained by thermal treatment of the sol-gel precursor at 500°C. The particle size was in agreement with the XRD measurements (12 nm), although the presence of aggregates was determined. The in-house prepared nanocrystalline titania was used as a filler in the preparation of Nafion based composite membranes to be used as electrolytes in PEMFCs, with the aim of increasing the operation temperature. The electrochemical performance of such membranes was investigated both in methanol and hydrogen fuelled fuel cells.
The strategy followed by researchers of NAST Centre is to develop organic/inorganic hybrid membranes in order to achieve the goal of obtaining PEMs capable to operate at temperatures up to and above 120°C . Organic/inorganic hybrids are investigated for a variety of applications, ranging from optics to electronics, to sensors and many others, since they offer the possibility to combine the properties of the two components in a unique material [4]. Thus, we have studied nanocomposites where inorganic materials were added to a polymeric matrix, either of Nafion or of totally aromatic sulfonated polymers. In the case of polymeric electrolytes, the addition of nanocrystalline oxides is effective in improving ionic conductivity, mechanical strength, and thermal stability [5]. Since performance is strongly influenced by the filler particle size, we developed a simple and versatile route for the preparation of ceramic oxides with nanometric grain size [6]. MxOy (M = Ti, Nb, In and Zr) were synthesized by rapid hydrolysis of an alcoholic solution of metal alkoxides. The preparative conditions were optimized to enhance the reaction kinetics leading to fast nucleation and favoring the formation of nanoparticles. Figure 2 shows the TEM micrograph of TiO2 (chosen as a representative example) obtained by thermal treatment of the sol-gel precursor at 500°C. The particle size was in agreement with the XRD measurements (12 nm), although the presence of aggregates was determined. The in-house prepared nanocrystalline titania was used as a filler in the preparation of Nafion based composite membranes to be used as electrolytes in PEMFCs, with the aim of increasing the operation temperature. The electrochemical performance of such membranes was investigated both in methanol and hydrogen fuelled fuel cells.
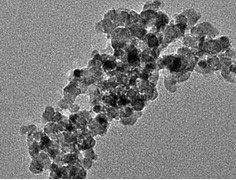
Figure 2. TEM micrograph of sol-gel derived Titania calcined at 500°C.
All the Membrane Electrode Assemblies (MEAs) prepared with the membranes of different composition were capable to operate in a DMFC up to 145°C, a temperature much higher than that reached by a bare Nafion recast membrane.
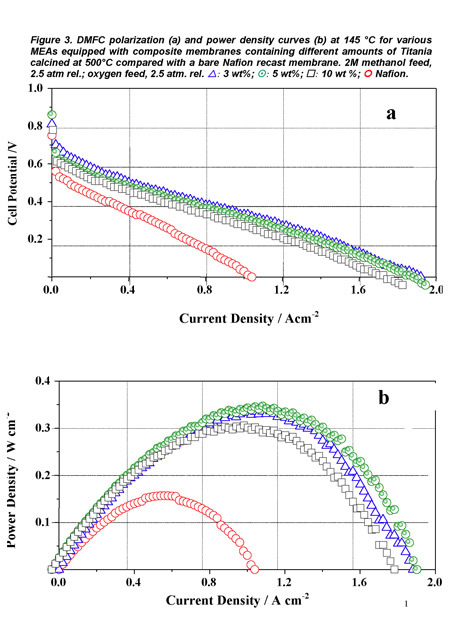
Figure 3 shows, again as a representative example, the polarization (a) and power density (b) curves obtained for the DMFC prepared with such MEAs with a 2 M methanol solution feed at the anode, and using oxygen as oxidant. A maximum power density of 350 mW cm-2 was reached at a current density of about 1.1 A cm-2 with the membrane containing 5 wt% TiO2. Moreover, the cell was subjected to a one month cycled operation (6 hours per day including start up and shut down procedures) without significant performance decrease. The presence of the fillers contributed to stabilize the polymer morphology at high temperature and inhibited the direct permeation of reaction gases by modifying the transport pathways [7].
 Operation at temperatures above 100°C is needed also for hydrogen-fuelled polymer electrolyte fuel cells, both for direct hydrogen (DH-PEFC) and processed hydrogen (PH-PEFC) cells, to enhance reaction kinetics, reduce heat-exchange requirements and, in PH-PEFC, increase CO tolerance. We have thus studied the electrochemical performance of a Nafion based composite membrane, containing 3 wt% nanometric TiO2 calcined at 400°C [8].
Operation at temperatures above 100°C is needed also for hydrogen-fuelled polymer electrolyte fuel cells, both for direct hydrogen (DH-PEFC) and processed hydrogen (PH-PEFC) cells, to enhance reaction kinetics, reduce heat-exchange requirements and, in PH-PEFC, increase CO tolerance. We have thus studied the electrochemical performance of a Nafion based composite membrane, containing 3 wt% nanometric TiO2 calcined at 400°C [8].
Table I summarizes the water uptake and ion exchange capacity (IEC) of the nanocomposite membrane compared with the values relative to a pure Nafion recast membrane prepared in the same experimental conditions and a commercial Nafion 115 membrane. The water uptake and ion exchange capacity of the composite membrane were significantly higher than those determined for the reference pure Nafion recast membrane, while commercial Nafion 115 gave intermediate values
Table I
Water uptake and ion exchange capacity data for different Nafion-based membranes.
| Membrane |
Water uptake (%) |
IEC (meq/g) |
| Nafion 115 |
27 |
0.91 |
| Nafion recast |
20 | 0.89 |
| Nafion-TiO2 |
29 |
0.93 |
The proton conductivity of the membranes was examined in the temperature range from 80°C to 130°C. The conductivity was always higher for the composite membrane, reaching a value of 0.18 S/cm at 130°C. The commercial Nafion 115 and the composite membrane were tested in DH-PEFC, in a single cell, in humidified H2/air, between 80°C and 130 °C. In the whole temperature range tested, the best performance was obtained with the composite membrane. Figure 4 shows the polarization and power
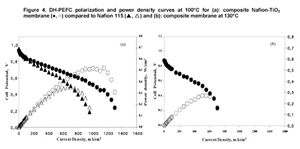 Arylene main chain polymers have receive a great deal of attention as possible alternative to perfluorinated systems mainly because of their lower cost and their ease of functionalization [9,10]. Among the different polymers, we focused our attention on sulfonated polyetheretherketone (SPEEK, Figure 6).density curves obtained for the two membranes at 100°C and those relative to the nanocomposite membrane at 130°C. A power density of 0.514 mW cm-2 was recorded for the composite membrane versus 0.354 mW cm-2 obtained with Nafion 115 at 0.56 V and 110 °C. Most important is to notice that while the pure Nafion membrane was damaged at temperatures above 100°C, the composite membrane continued to operate up to 130°C reaching a power density of 0.254 mW cm-2 at 0.5 V. Tests were also carried out in a PH-PEFC. The cell was fed with steam reforming synthetic fuel (SR: 10 ppm CO; 20% CO2; 75% H2; 1% CH4). Figure 5 shows a comparison between polarization curves at 110°C in pure hydrogen and SR for the nanocomposite membrane. For both fuels, the measured OCV values were good, almost reaching 1 V. A power density value of about 182 mW cm-2 at 0.6 V in synthetic fuel, versus the 366 mW cm-2 value measured in pure hydrogen, was obtained.
Arylene main chain polymers have receive a great deal of attention as possible alternative to perfluorinated systems mainly because of their lower cost and their ease of functionalization [9,10]. Among the different polymers, we focused our attention on sulfonated polyetheretherketone (SPEEK, Figure 6).density curves obtained for the two membranes at 100°C and those relative to the nanocomposite membrane at 130°C. A power density of 0.514 mW cm-2 was recorded for the composite membrane versus 0.354 mW cm-2 obtained with Nafion 115 at 0.56 V and 110 °C. Most important is to notice that while the pure Nafion membrane was damaged at temperatures above 100°C, the composite membrane continued to operate up to 130°C reaching a power density of 0.254 mW cm-2 at 0.5 V. Tests were also carried out in a PH-PEFC. The cell was fed with steam reforming synthetic fuel (SR: 10 ppm CO; 20% CO2; 75% H2; 1% CH4). Figure 5 shows a comparison between polarization curves at 110°C in pure hydrogen and SR for the nanocomposite membrane. For both fuels, the measured OCV values were good, almost reaching 1 V. A power density value of about 182 mW cm-2 at 0.6 V in synthetic fuel, versus the 366 mW cm-2 value measured in pure hydrogen, was obtained.
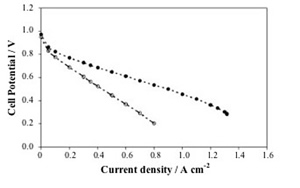 Although the performance for tested MEA fed with SR is lower by about 50% if compared to the performance in pure hydrogen, it is reasonable to assume that the use of suitable electrodes containing Pt-Ru as an electro-catalyst would minimize such loss. Nevertheless, it appeared evident that the filler exerts a beneficial effect in allowing operation at T > 100 °C even in SR fuel.
Although the performance for tested MEA fed with SR is lower by about 50% if compared to the performance in pure hydrogen, it is reasonable to assume that the use of suitable electrodes containing Pt-Ru as an electro-catalyst would minimize such loss. Nevertheless, it appeared evident that the filler exerts a beneficial effect in allowing operation at T > 100 °C even in SR fuel.
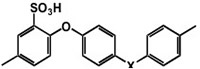
Figure 6 The structure of sulfonated Poly Ether Ether Ketone (SPEEK)
 Even if at high degree of sulfonation (DS), its conductivity is large enough to meet the requirements needed for application in PEMFCs, its mechanical properties tend to progressively deteriorate with sulfonation because of the absence of significant hydrophilic-hydrophobic separation that results in very narrow and poorly connected water channels and large separation between the sulfonic acid groups [10,11]. To attain the correct balance between the hydrophilic and hydrophobic components we prepared nancomposite membranes containing hydrated tin oxide, a filler that is proton conducting in its own right and to decrease the polymer DS.
Even if at high degree of sulfonation (DS), its conductivity is large enough to meet the requirements needed for application in PEMFCs, its mechanical properties tend to progressively deteriorate with sulfonation because of the absence of significant hydrophilic-hydrophobic separation that results in very narrow and poorly connected water channels and large separation between the sulfonic acid groups [10,11]. To attain the correct balance between the hydrophilic and hydrophobic components we prepared nancomposite membranes containing hydrated tin oxide, a filler that is proton conducting in its own right and to decrease the polymer DS.
The procedure used to synthesize SnO2 x 1.5H2O allowed to obtain nanosized particles. Figure 7 shows the typical SEM micrograph of the as prepared SnO2 x n(H2O) powder. As typical morphology for hydrated compounds, the powder was made of soft agglomerates. Numerous SEM observations allowed to measure the unit particles in the nanometric size range (5-15 nm). Such a small particle size is expected to ease the formation of homogeneous composite membranes.
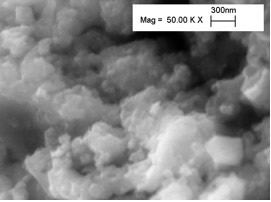
Figure 7. SEM micrograph of the as-prepared SnO2 x nH2O powder.
Figure 8 shows the Arrhenius plots of the conductivity of the composite membranes and of the unfilled SPEEK reference membrane. In the region between 25°C and 75°C, the proton conductivity (σ) values of the composite membrane were always larger than the values measured for the unfilled SPEEK membrane. In particular, at 25°C, σ of the composite membrane was one order of magnitude larger than the σ of the unfilled SPEEK (composite: 0.016 S cm-1; unfilled SPEEK 0.0017 S cm-1). This difference decreased with increasing the temperature and eventually was null at 75°C. The λ values (the number of water molecules per sulfonic acid group of SPEEK) was calculated on the basis of water uptake measurements and considering an additive behavior of the components.
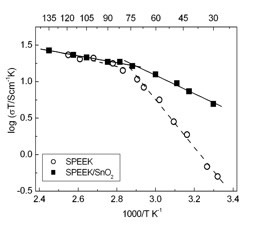
Figure 8. Arrhenius plots of the unfilled SPEEK and SPEEK/SnO2 composite membranes at 100% RH.
In agreement with electrochemical data, λ of the composite membrane was larger than the λ value of the SPEEK membrane below 60°C, temperature at which the λ values of the two samples became the same.
The overall reduced swelling of the composite membrane can be explained assuming that the filler induces morphological modification of the membrane and that the oxide hydration water molecules generate a connection between the polymer sulfonic acid moieties, creating different hydrophilic paths that favor proton transfer [12]. The effect becomes negligible at higher temperature when a decrease in the interactions between polymer chains is known to favor a greater hydration of the polymer [13]. The reduced swelling and higher morphological stability of the composite resulted also in improved membrane performance in terms of durability.
The electrochemical performance of the membranes was tested in a home-made prototype DMFC single cell (Figure 9), acquiring polarization curves.
 Figure 9. The H2 and CH3OH fuelled Fuel Cell built at Tor Vergata.
Figure 9. The H2 and CH3OH fuelled Fuel Cell built at Tor Vergata.
Figure 10 shows the polarization and power curves for the SPEEK/SnO2 composite membrane in a DMFC test at 100 °C, compared with a recast Nafion membrane. As already mentioned, Nafion is the most widely used electrolyte for DMFCs, even though it exhibits high methanol permeation rate as well as a drop in proton conductivity at temperatures larger than 80°C. The I-V curves clearly showed the improved performance of the composite membrane with respect to the Nafion membrane. The open circuit voltage (OCV) for the composite membrane (0.70 V) was larger than that of unfilled SPEEK (0.65 V) and reference Nafion (0.59 V). The polarization curve of the unfilled SPEEK membrane is not shown due to its lower stability in terms of proton conductivity as a function of time. This finding shows that the use of a SPEEK membrane allowed to reduce the methanol crossover with respect to Nafion. Moreover, the presence of tin oxide allowed a further reduction of the methanol crossover.
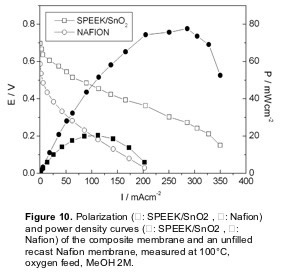 In the whole voltage range investigated, the current values of the SPEEK/SnO2 membrane were always larger than the values obtained with the Nafion membrane. In particular, the composite membrane reached a maximum current density value of 350 mAcm-2 (with respect to 200 mAcm-2 for unfilled Nafion). The maximum power density value reached at 100°C with the composite membrane was 80 mWcm-2 at a current density of about 300 m A cm-2, whereas the maximum power density value of the reference unfilled Nafion membrane at the same temperature was 20 mWcm-2, at a current density of about 120 mAcm-2. The improvement of the polarization curve of the SPEEK/SnO2 composite membrane reflected the drop in conductivity of the unfilled Nafion membrane above 90°C and the enhanced stability of the composite membrane at 100°C.
In the whole voltage range investigated, the current values of the SPEEK/SnO2 membrane were always larger than the values obtained with the Nafion membrane. In particular, the composite membrane reached a maximum current density value of 350 mAcm-2 (with respect to 200 mAcm-2 for unfilled Nafion). The maximum power density value reached at 100°C with the composite membrane was 80 mWcm-2 at a current density of about 300 m A cm-2, whereas the maximum power density value of the reference unfilled Nafion membrane at the same temperature was 20 mWcm-2, at a current density of about 120 mAcm-2. The improvement of the polarization curve of the SPEEK/SnO2 composite membrane reflected the drop in conductivity of the unfilled Nafion membrane above 90°C and the enhanced stability of the composite membrane at 100°C.
These results converge to indicate that filling SPEEK membranes with hydrated tin oxide allowed to improve the membrane stability, as well as to decrease its methanol permeability. These features led to an enhancement of cell performance of the composite membrane with respect to our reference Nafion recast membrane, pointing out that SPEEK/SnO2 membrane is a promising electrolyte for DMFCs.
Conclusions
The use of organic/inorganic hybrid membranes has been demonstrated to be effective in achieving good electrochemical performance of PEMFCs at temperatures above 120°C, fed either with processed hydrogen, steam-reformed hydrogen, and methanol.
This research represents some of the results of collaborative efforts of different Italian and foreign Institutions under the framework of MiUR-FISR, MAE-Joint Laboratory and EU-Coordinative Action Projects.
Co-authors of the study
S. Licoccia, E. Traversa Centro NAST & DSTC, Università di Roma Tor Vergata
A. D’Epifanio, C. D’Ottavi B. Mecheri, R. Polini DSTC Università di Roma Tor Vergata
P. Antonucci Univ. Mediterranea of Reggio Calabria
V. Antonucci, A. S. Aricò, E. Passalacqua V. Baglio, A. Carbone, A. Di Blasi, A. Saccà, R. Ornelas CNR-TAE Messina
References
[1] K. A. Mauritz, R. B. Moore, Chem. Rev. 104 (2004) 4535.
[2] M. A. Hickner, H. Ghassemi, Y. S. Kim, B. R. Einsla, J. E. McGrath, Chem. Rev. 104 (2004) 4587.
[3] C. Wieser, Fuel Cells 4 (2004) 245.
[4] C. Sanchez, G. J. De A.A. Soler-Illia, F. Ribot, D. Grosso, C. R. Chimie 6 (2003) 1131.
[5] S. Licoccia, E. Traversa, in: H.-J. Fecht, M. Werner (Eds.) The Nano-Micro Interface: Bridging the Micro and Nano Worlds Together, Wiley-VCH Verlag, Weinheim, 2004, pp.289-301.
[6] S. Licoccia, R. Polini, C. D’Ottavi, F. Serraino Fiory, M.L. Di Vona, E. Traversa, J. Nanosci. Nanotechnol. 5 (2005) 592.
[7] D. J. Jones, J. Rozière, in W. Weslich, A. Lamm, H. A. Gasteiger (eds.), Handbook of Fuel Cells, Fundamental Technology and Applications, vol. 3, Wiley, 2003, pp. 447-455.
[8] A. Saccà, A. Carbone, E. Passalacqua, A. D’Epifanio, S. Licoccia, E. Traversa, E. Sala, F. Traini, R. Ornelas, J. Power Sources (2005) in press.
[9] B. Mecheri, A. D’Epifanio, E. Traversa, S. Licoccia, J. Power Sources, 169 (2007) 247–252
[10] P. Krishnan, J. Park, T. Yang, W. Lee, C. Kim, J. Power Sources, 163 (2006) 2-8
[11] M. Cappadonia, J. W. Erning, S. M. S. Niaki, U. Stimming, Solid State Ionics, 77 (1995) 65-69
[12] V. Ramani, H. R. Kunz, J. M. Fenton, J. Membr. Sci., 232 (2004) 31–44
[13] D. J. Jones, J. Rozière, Ann. Rev. Mater. Res. 33 (2003)
Related Information
Prof. S. Licoccia
NUME, CARISMA, CNR-TAE,
Università Mediterranea Reggio Calabria,
RCAST Tokyo, Università di Roma Tor Vergata


