 In stark contrast to the crystalline state, amorphous material display a complete lank of long range structural order. In the case of ice it came as a huge surprise that several different amorphous ices exist and they have been classified according to their densities. The waters molecules in ice are held together through hydrogen bonds and we have set aut to investigate how changes in density affect hydrogen bonding and nuclear quantum effects of the hydrogen atoms.
In stark contrast to the crystalline state, amorphous material display a complete lank of long range structural order. In the case of ice it came as a huge surprise that several different amorphous ices exist and they have been classified according to their densities. The waters molecules in ice are held together through hydrogen bonds and we have set aut to investigate how changes in density affect hydrogen bonding and nuclear quantum effects of the hydrogen atoms.
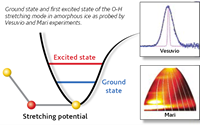
Date from the Vesuvio instruments showed a pronounced softening of the O-H stretching potential in the low-density ice compared with the higher- density ices. Moreover, the hydrogen mean kinetic energy was found to increase with increasing density indicating the weakening of hydrogen bonds as well as steeper and more harmonic hydrogen vibrational potential energy surface. In a novel approach, we used data from both the Vesuvio and Maria instruments to determine the anharmonicity constants of the O-H stretching modes. We then arrived at a very simple conclusion : hydrogen banding is the main cause for anharmonicity in amorphous ice.
Evolution :A. Parmentier ( Università degli Studi di Roma Tor Vergata, Italy), JJ Shephard ( University College London and Durham University), G. Romanelli ( Università degli Studi di Roma Tor Vergata, Italy, and ISIS), R. Senesi (Università degli Studi di Roma Tor Vergata and CNR-IPCF sezione di Messina, Italy), C.G. Salzmann (University College London) C. Andreani ( Universita degli Studi di Roma Tor Vergata, Italy).
Research Support:
Royal Society ( CGS, UF 100144); Leverhulme Trust ( RPG – 2014 – 04);
CNR – ISIS Agreement (2014-2020)
Contact:
Dr. G. Romanelli, giovanni.romanelli@stfc.ac.uk
Dr. C.G. Salzmann , c.salzmann@url.ac.uk
Further information:
A. Parmentier et al. J. Phys. Chem. Lett., 6 (2015) 2038-2042


