- Home
- research-infrastructure
Life Science
“Aggregation of peptides in neurodegenerative diseases”
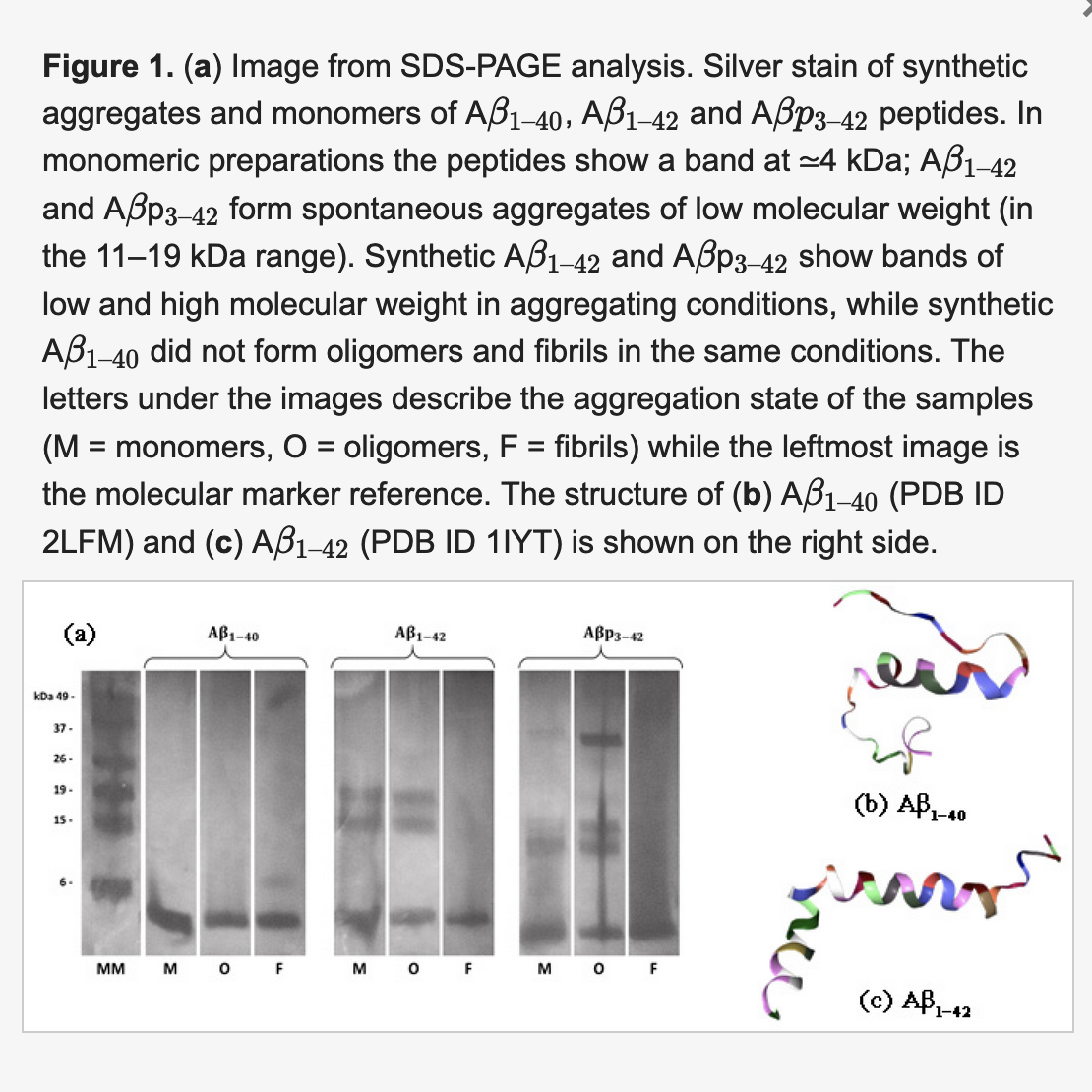
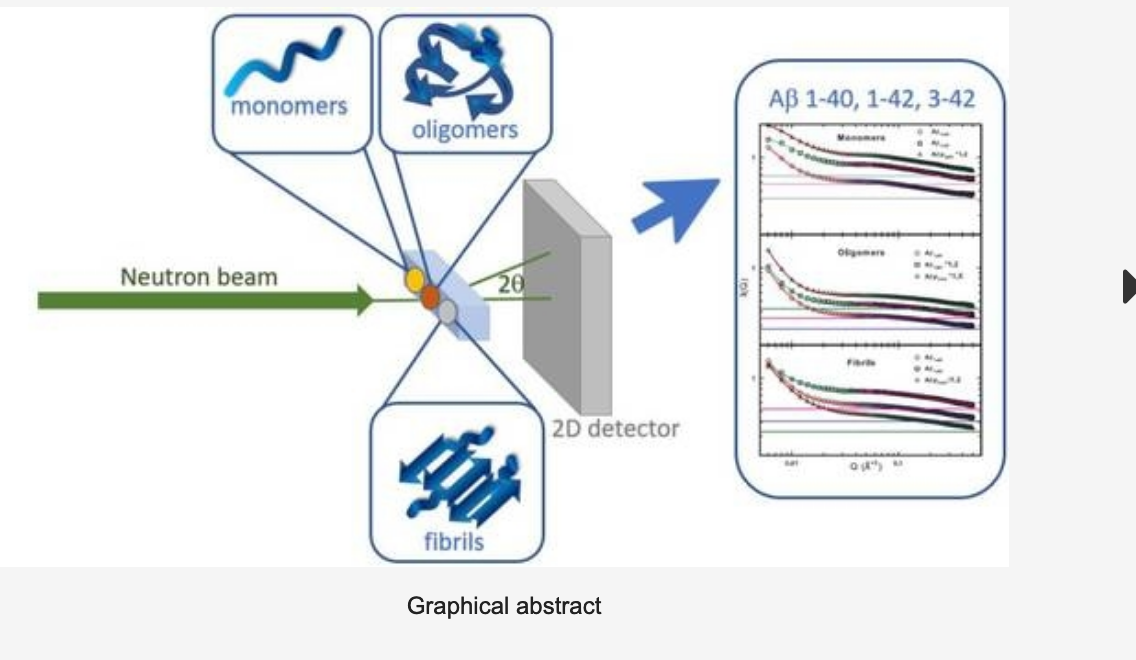 One of the global challenges for the treatment of neurodegenerative diseases is the understanding of the microscopic mechanisms of aggregation of beta-amyloids, which are the peptides responsible for the formation of neuritic plaques observed in the brains of Alzheimer’s patients. In the last three years the UTV team has studied the behavior of amyloid peptides in vitro with electron microscopy and atomic force techniques, UV-VIS and X-ray spectroscopy and has designed and realized neutron scattering characterizations at ISIS using the Small Angle Neutron Scattering (SANS) beam line. Aggregation states of amyloid beta peptides for amyloid beta A β 1–40 to A β 1–42 and A β p 3–42 have been investigated through SANS, a technique which allows to study the size and fractal nature of the monomers, oligomers and fibrils of the three different peptides. The knowledge of these small peptides and their aggregation state are of key importance for the comprehension of neurodegenerative diseases (e.g., Alzheimer’s disease). Results showed that all the investigated peptides have monomers with a radius of gyration of the order of 10 Å, while the oligomers and fibrils display differences in size and aggregation ability, with A β p 3–42 showing larger oligomers. These properties are strictly related to the toxicity of the corresponding amyloid peptide and indeed to the development of the associated disease. Through the above mentioned complementary characterization it was possible to characterize the biophysical properties and the states of aggregation of beta amyloids, and to understand their behavior in different solvation environments. Following the creation of the node, the UTV group plans to expand these research and development activities on the aggregation mechanisms of beta-amyloids, which envisages experimental analyzes to be realized- through the platform ‘apply for beamtime” – both at ISIS@MACH, and at ISIS facility, using complementary neutron techniques at ISIS neutron beamlines IRIS, OSIRIS, MAPS, WISH, OffSpec beam lines, Larmor,SANS and SANS2D.
One of the global challenges for the treatment of neurodegenerative diseases is the understanding of the microscopic mechanisms of aggregation of beta-amyloids, which are the peptides responsible for the formation of neuritic plaques observed in the brains of Alzheimer’s patients. In the last three years the UTV team has studied the behavior of amyloid peptides in vitro with electron microscopy and atomic force techniques, UV-VIS and X-ray spectroscopy and has designed and realized neutron scattering characterizations at ISIS using the Small Angle Neutron Scattering (SANS) beam line. Aggregation states of amyloid beta peptides for amyloid beta A β 1–40 to A β 1–42 and A β p 3–42 have been investigated through SANS, a technique which allows to study the size and fractal nature of the monomers, oligomers and fibrils of the three different peptides. The knowledge of these small peptides and their aggregation state are of key importance for the comprehension of neurodegenerative diseases (e.g., Alzheimer’s disease). Results showed that all the investigated peptides have monomers with a radius of gyration of the order of 10 Å, while the oligomers and fibrils display differences in size and aggregation ability, with A β p 3–42 showing larger oligomers. These properties are strictly related to the toxicity of the corresponding amyloid peptide and indeed to the development of the associated disease. Through the above mentioned complementary characterization it was possible to characterize the biophysical properties and the states of aggregation of beta amyloids, and to understand their behavior in different solvation environments. Following the creation of the node, the UTV group plans to expand these research and development activities on the aggregation mechanisms of beta-amyloids, which envisages experimental analyzes to be realized- through the platform ‘apply for beamtime” – both at ISIS@MACH, and at ISIS facility, using complementary neutron techniques at ISIS neutron beamlines IRIS, OSIRIS, MAPS, WISH, OffSpec beam lines, Larmor,SANS and SANS2D.
References
Stellato, F., Fusco, Z., Chiaraluce, R., Consalvi, V., Dinarelli, S., Placidi, E., … & Morante, S. (2017). The effect of β-sheet breaker peptides on metal associated Amyloid-β peptide aggregation process. Biophysical chemistry, 229, 110-114.
De Santis, E., Minicozzi, V., Proux, O., Rossi, G., Silva, K. I., Lawless, M. J., … & Morante, S. (2015). Cu (II)–Zn (II) Cross-Modulation in Amyloid–Beta Peptide Binding: An X-ray Absorption Spectroscopy Study. The Journal of Physical Chemistry B, 119 (52), 15813-15820.
Stellato, F., Minicozzi, V., Millhauser, G. L., Pascucci, M., Proux, O., Rossi, G. C., et al. & Morante, S. (2014). Copper–zinc cross-modulation in prion protein binding. European Biophysics Journal, 43(12), 631-642.
G. Festa, F. Mallamace, G. Sancesario, C. Corsaro, D. Mallamace, E. Fazio, L. Arcidiacono, V. Garcia Sakai, R. Senesi, E. Preziosi, G. Sancesario, C. Andreani, Aggregation states of Aβ1−40, Aβ1−42 and Aβp3−42 amyloid beta peptides: a SANS study, Int. J. Mol. Sci. 2019, 20, 4126; doi:10.3390/ijms20174126
G. Festa, G. Sancesario, C. Corsaro, S. Longo, D. Mallamace, E. Fazio, L. Arcidiacono, V. Garcia Sakai, R. Senesi, G. Sancesario, F. Mallamace, C. Andreani, SANS study of Amyloid beta1−40: unfolded monomers in DMSO, multidimensional aggregates in water medium, Physica A 517 (2019) 385–391
Life Science
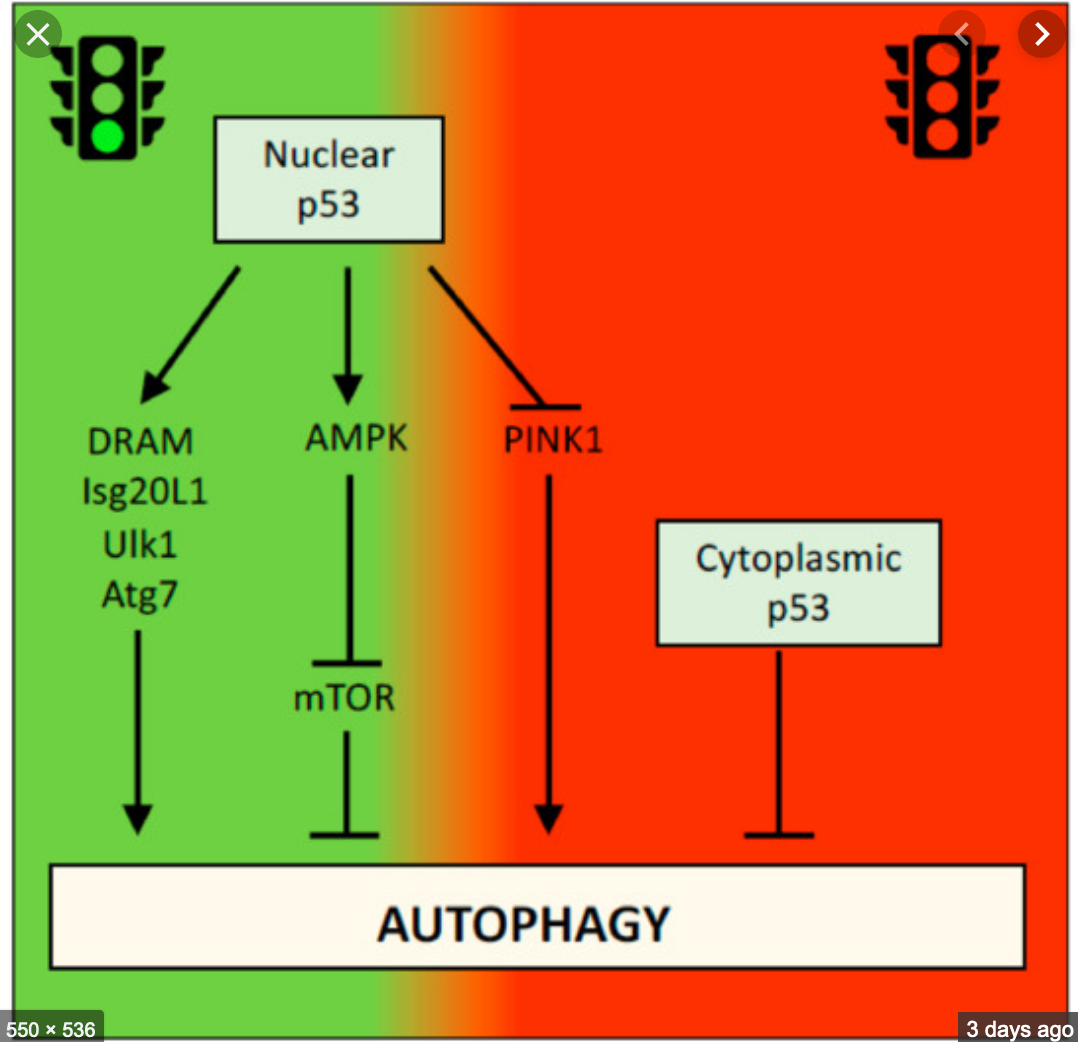 The p53 gene family includes three genes (p53, p63 and p73) that encode transcription factors whose activities are functionally associated with the pathogenesis of multiple human malignancies. Over the past three years, the UTV group has studied the molecular mechanisms by which these factors regulate the onset and progression of different tumors of epithelial origin, such as lung adenocarcinoma and squamous skin carcinomas. Using in vitro cellular models and mouse models, we have characterized the role of these factors in regulating different transcriptional programs involved in the remodeling of the extracellular matrix, in angiogenesis and in the regulation of cellular metabolism. We have also identified some epigenetic factors that regulate the transcriptional activity of the various members of this family in tumors. Following the creation of the ISIS@MACH, the UTV group plans to expand these research activities by focusing its research on how the pro-anti-tumor functions of the various family members are influenced by the biomechanical and physical properties of the extracellular matrix. We intend to launch a new characterization of the ‘biomaterials’, which foresees experimental analyzes to be realized at ISIS@MACH – through platform for “apply for beamtime” – and at ISIS neutron beam lines IRIS, OSIRIS, WISH, OffSpec, Larmor, SANS, SANS2D.
The p53 gene family includes three genes (p53, p63 and p73) that encode transcription factors whose activities are functionally associated with the pathogenesis of multiple human malignancies. Over the past three years, the UTV group has studied the molecular mechanisms by which these factors regulate the onset and progression of different tumors of epithelial origin, such as lung adenocarcinoma and squamous skin carcinomas. Using in vitro cellular models and mouse models, we have characterized the role of these factors in regulating different transcriptional programs involved in the remodeling of the extracellular matrix, in angiogenesis and in the regulation of cellular metabolism. We have also identified some epigenetic factors that regulate the transcriptional activity of the various members of this family in tumors. Following the creation of the ISIS@MACH, the UTV group plans to expand these research activities by focusing its research on how the pro-anti-tumor functions of the various family members are influenced by the biomechanical and physical properties of the extracellular matrix. We intend to launch a new characterization of the ‘biomaterials’, which foresees experimental analyzes to be realized at ISIS@MACH – through platform for “apply for beamtime” – and at ISIS neutron beam lines IRIS, OSIRIS, WISH, OffSpec, Larmor, SANS, SANS2D.References (TO BE ADDED)
Life Science
“Analysis of brain networks”
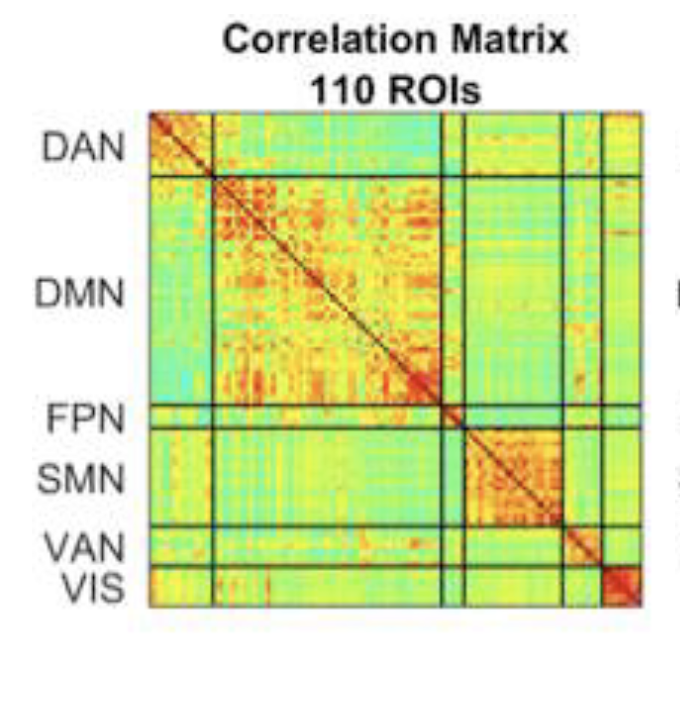 An essential requirement for the characterization of brain networks is the development of adequate methods of analysis in degrees of separate the non-specific contribution of physiological noise (related to breathing, heartbeat, etc.) from long-range correlation brain activity that defines functional networks. While spurious correlations induced by extraneural causes have sufficient distinctive elements (for example, they tend to be global) it is extremely difficult to separate them from functional correlations, especially if these are not static, but modulated by external factors (environmental stimulations, pathologies). In the last three years the Centro Fermi group has been developing advanced methods for the characterization and removal of physiological noise from fMRI data, exploiting both postprocessing methods and methods based on particular MRI techniques (for example, multi-echo acquisition). Following the creation of the node, the group plans to expand this research on brain networks, and start a new characterization program for ‘biomaterials’, which envisages project proposals to be realized – through the platform for “apply for beamtime” – both at ISIS @ MACH, and at ISIS, using the complementary ISIS neutron techniques at MAPS, WISH, OffSpec, Larmor beam lines, SANS, SANS2D.
An essential requirement for the characterization of brain networks is the development of adequate methods of analysis in degrees of separate the non-specific contribution of physiological noise (related to breathing, heartbeat, etc.) from long-range correlation brain activity that defines functional networks. While spurious correlations induced by extraneural causes have sufficient distinctive elements (for example, they tend to be global) it is extremely difficult to separate them from functional correlations, especially if these are not static, but modulated by external factors (environmental stimulations, pathologies). In the last three years the Centro Fermi group has been developing advanced methods for the characterization and removal of physiological noise from fMRI data, exploiting both postprocessing methods and methods based on particular MRI techniques (for example, multi-echo acquisition). Following the creation of the node, the group plans to expand this research on brain networks, and start a new characterization program for ‘biomaterials’, which envisages project proposals to be realized – through the platform for “apply for beamtime” – both at ISIS @ MACH, and at ISIS, using the complementary ISIS neutron techniques at MAPS, WISH, OffSpec, Larmor beam lines, SANS, SANS2D.References (TO BE ADDED)
Life Science
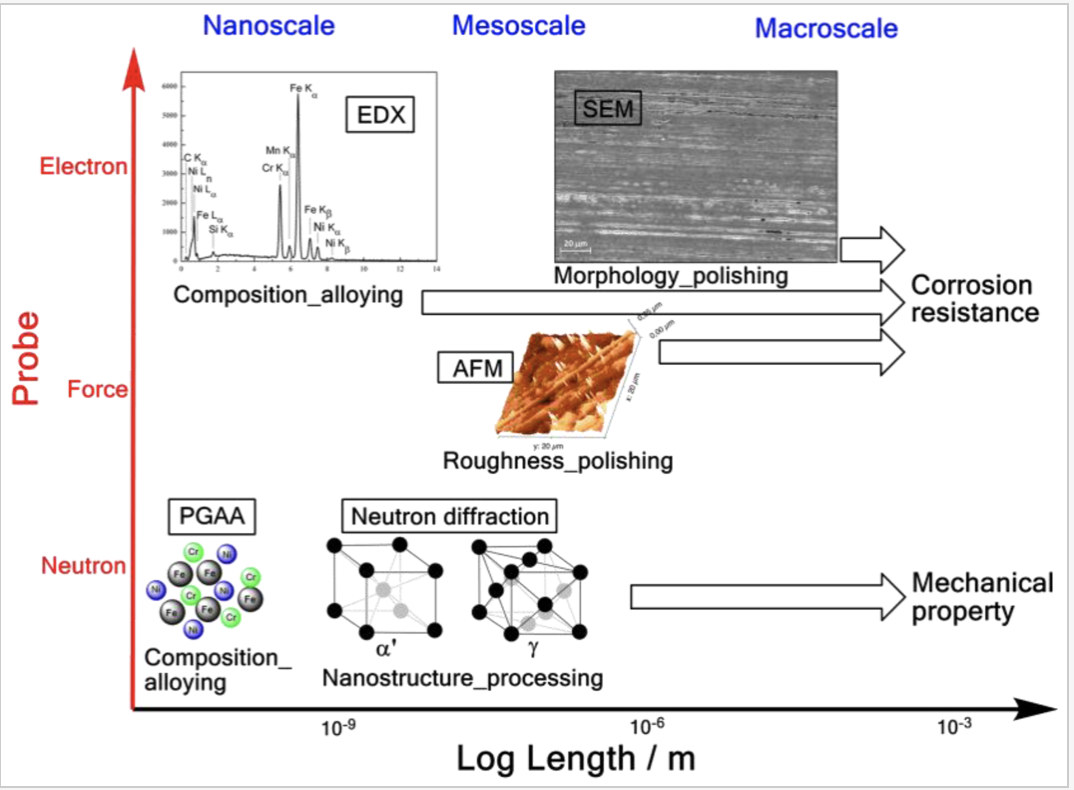 The control of the chemical, mechanical and biocompatibility properties of materials is a key element for the applications of composite materials to dentistry. In the last three years, the group of the NAST Center of UTV has carried out characterizations with PGAA (promp gamma activation analysis) techniques resolved in time, developed at the ISIS VESUVIO and INES beamlines, of the chemical, isotopic composition, structure and phases crystalline orthodontic wires allows to determine the degree of reproducibility of clinical performance following implantation. Using innovative techniques, the group of the NAST Center of UTV carried out characterizations of the materials both in the laboratories of UTV and ISIS, which allowed to determine the microscopic structure and the chemical composition of metal wires for orthodontics, in the context of standardization requirements and marking required in Europe. Following the creation of the node, the UTV group plans to expand these development and development activities on metal for orthodontics, carrying out both the design of new characterizations and the analysis of the chemical, mechanical and biocompatibility properties of the materials. We intend to start characterization of the material, which provides for new experimental analyses to be realized- through the platform ” apply for beamtime” – at ISIS@MACH, and at ISIS, using the complementary ISIS neutron techniques of the VESUVIO, INES, ENGIN-X and IMAT beam lines.
The control of the chemical, mechanical and biocompatibility properties of materials is a key element for the applications of composite materials to dentistry. In the last three years, the group of the NAST Center of UTV has carried out characterizations with PGAA (promp gamma activation analysis) techniques resolved in time, developed at the ISIS VESUVIO and INES beamlines, of the chemical, isotopic composition, structure and phases crystalline orthodontic wires allows to determine the degree of reproducibility of clinical performance following implantation. Using innovative techniques, the group of the NAST Center of UTV carried out characterizations of the materials both in the laboratories of UTV and ISIS, which allowed to determine the microscopic structure and the chemical composition of metal wires for orthodontics, in the context of standardization requirements and marking required in Europe. Following the creation of the node, the UTV group plans to expand these development and development activities on metal for orthodontics, carrying out both the design of new characterizations and the analysis of the chemical, mechanical and biocompatibility properties of the materials. We intend to start characterization of the material, which provides for new experimental analyses to be realized- through the platform ” apply for beamtime” – at ISIS@MACH, and at ISIS, using the complementary ISIS neutron techniques of the VESUVIO, INES, ENGIN-X and IMAT beam lines.References
Kun V. Tian, Francesca Passaretti, Adelaide Nespoli, Ernesto Placidi, Carla Andreani, Silvia Licoccia, Roberta Condò, Gregory A. Chass, Roberto Senesi and Paola Cozza, “Composition-nanostructure predictions of performance in stainless steel archwires”, Nanomaterials 9, 1119 (2019).
K. V. Tian, G. Festa, L. Szentmiklósi, B. Maróti, L. Arcidiacono, G. Laganà, C. Andreani, S. Licoccia, R. Senesi, P. Cozza, ‘Compositional studies of functional orthodontic archwires using prompt-gamma activation analysis at a pulsed neutron source vs. a cold neutron source’, Journal of Analytical Atomic Spectrometry, 7, 1-8, (2017).
K. V. Tian, G. Festa, F. Basoli, G. Laganà, A. Scherillo, C. Andreani, S. Licoccia, R. Senesi and P. Cozza, ‘Orthodontic archwire composition and phase analyses by neutron spectroscopy’, Dental Materials Journal, 2017 May 31; 36(3):282-288. doi: 10.4012/dmj.2016-206, IF= 3.931.


